Pig kidney transplant in human successful in US
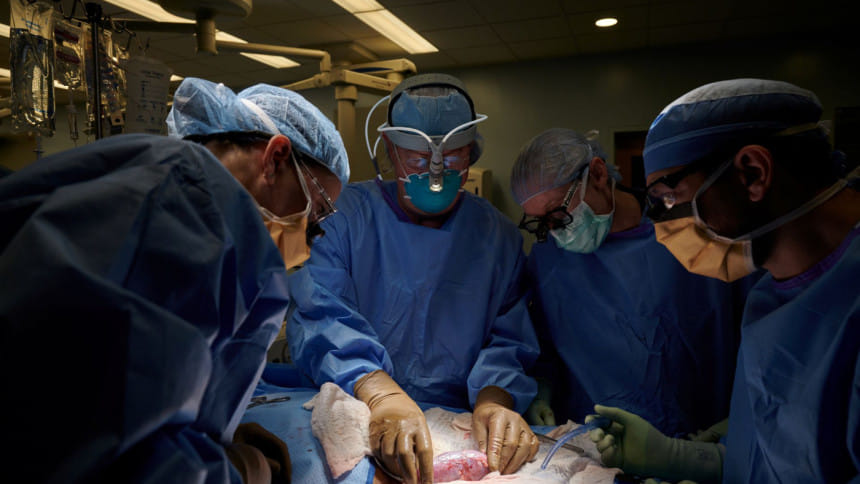
For the first time, a pig kidney has been transplanted into a human without triggering immediate rejection by the recipient's immune system, a potentially major advance that could eventually help alleviate a dire shortage of human organs for transplant.
The procedure, performed at NYU Langone Health in New York City, involved the use of a pig whose genes had been changed so that its tissues no longer contained a molecule known to trigger almost immediate rejection.
Researchers told Reuters that the recipient was a brain-dead patient with symptoms of kidney dysfunction, whose family consented to the experiment before he was taken off life support.
For three days, the new kidney was attached to his blood vessels and placed outside his body, giving researchers access to it.
The test results of the transplanted kidney function "looked quite normal," said transplant surgeon Dr. Robert Montgomery, who led the study.
The kidney produced "the amount of urine you would expect" from a transplanted human kidney, he said, and there was no evidence of vigorous, early rejection when the undigested pig kidney is transplanted into non-human primates.
Montgomery said the recipient's abnormal creatinine level — an indicator of poor kidney function — returned to normal after the transplant.
In the United States, approximately 107,000 people are currently awaiting organ transplants, with more than 90,000 waiting for a kidney, according to the United Network for Organ Sharing. A kidney waits an average of three to five years.
Researchers have been working on the possibility of using animal organs for transplants for decades, but have held off on how to prevent immediate rejection by the human body.
Montgomery's team theorized that knocking out the pig's gene for a carbohydrate that triggers rejection — a sugar molecule, or glycan, called an alpha-gal — would stop the problem.
The genetically modified pig, called GalSafe, was developed by the United Therapeutics Corp's (UTHR.O) Revivicor Unit. It was approved by the US Food and Drug Administration in December 2020 for use as a food for people with meat allergies and as a potential source of human therapeutics.
The agency said medical products developed from pigs would still require specific FDA approval before they could be used in humans.
Other researchers are considering whether GalSafe pigs could be the source of everything from heart valves to skin grafts for human patients.
The NYU kidney transplant experiment should pave the way for trials in patients with end-stage kidney failure, possibly in the next year or two, said Montgomery, himself a heart transplant recipient. Those trials may test the approach as a short-term solution for critically ill patients until a human kidney becomes available, or as a permanent graft.
Montgomery said the current experiment involved a single transplant, and the kidney was left in place for only three days, so any future trials are likely to uncover new obstacles that will need to be overcome. Participants will probably be patients who have a low probability of receiving a human kidney and a poor prognosis on dialysis.
"For a lot of them, the death rate is as high as it is for some cancers, and we don't think twice about using new drugs and doing new tests[in cancer patients]when it comes to them." A few months can give more of life," Montgomery said.
Montgomery said the researchers worked with medical ethicists, legal and religious experts to examine the concept before asking a family for temporary access to a brain-dead patient.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 




Comments